Hydroarylation of internal alkynes by costeffective CoIII-catalysis, directed by N-tert-butyl amides, is achieved to avail mono- or dihydroarylated amide products selectively in an atom and step economic way. Several important functional groups were tolerated under the reaction conditions, and syn-hydroarylation products were exclusively isolated. Notably, a 4-fold C−H hydroarylation provided a highly conjugated organic framework in one step. Kinetic study with extensive deuterium labeling experiments were performed to support the proposed mechanism.
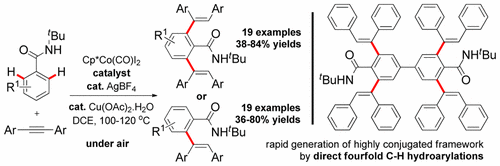
Title: Cp*CoIII−Catalyzed syn-Selective C−H Hydroarylation of Alkynes Using Benzamides: An Approach Toward Highly Conjugated Organic Frameworks
Journal: J. Org. Chem. 2017, 82, 420−430
